Solving compliance and international manufacturing challenges
Zimmer Biomet - USA
Contact us

Founded in 1927 to produce aluminum splints - cutting edge at the time - Zimmer Biomet is a medical device company commanding second place in the entire world’s overall orthopedic market share. The organization’s stated purpose is to “Restore mobility, alleviate pain, and improve the quality of life for patients around the world.” For at least one Zimmer Biomet quality engineer, the nature of the company’s products and what it takes to manufacture them takes on a decidedly sensitive tone.
 “For us, yes, we make great products, and yes, our company has a good stock price, but when you see a patient walking around with your product in them, it’s pretty compelling,” says Jeff Livingston, Senior Quality Engineer at the Zimmer Biomet complex in Warsaw, Indiana. “I’ve done some pretty cool things in automotive, but what we do here is huge. Mostly it’s about regular folks just trying to live again, trying to get through life without pain.”
“For us, yes, we make great products, and yes, our company has a good stock price, but when you see a patient walking around with your product in them, it’s pretty compelling,” says Jeff Livingston, Senior Quality Engineer at the Zimmer Biomet complex in Warsaw, Indiana. “I’ve done some pretty cool things in automotive, but what we do here is huge. Mostly it’s about regular folks just trying to live again, trying to get through life without pain.”
Not only does Zimmer Biomet do cool and compelling things, it does them on multiple continents. Naturally, the logistics of running an international high-tech manufacturing firm are complex, and the challenges of regulatory compliance require the most rigorous controls and comprehensive solutions.
Critical tolerances
Although the aerospace community may define manufacturing tolerances in terms of ten-thousandths of an inch, and the orthopedic implant industry expresses in μm or micrometers, both fields deal in tolerances that are critical to the performance of a larger system. Implants do, however, carry additional ramifications.
“We make orthopedic implants; if you get a bad implant, you don’t just pull over to the side of the road and change it,” quips Livingston. “It’s a difficult deal for everybody. Measurements on these products are critical.”
Methods for lean manufacturing
“For QC and FDA compliance, we used to use temperature humidity monitoring (THUM) devices and log books to monitor the conditions at our coordinate measuring machines (CMM),” says Livingston. “The problem was that when conditions exceeded the allowable range, the THUM would power down the computer itself. We had to reboot the computer and then reboot the CMM. It could take 35 minutes to recover, and we would lose all the measurement data at the time—which is a primary thing. Ultimately, it was too complex, and there was too much inherent risk.”
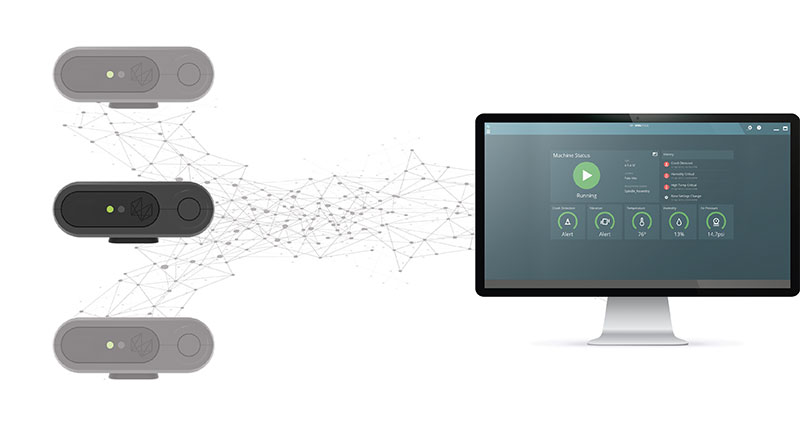
To manufacture high-quality products and achieve the requisite critical tolerances, Zimmer Biomet now incorporates PULSE systems on its CMMs.
Designed by Hexagon Manufacturing Intelligence to ensure data integrity while also improving safety and security in modern shop-floor inspection conditions, PULSE uses a network of sensors to record variations in temperature, vibration, CMM collisions, and humidity in the CMM’s area of operation. The PULSE system creates a central dashboard of information for operators to access at any time, and offers equipment status alerts and crash notifications that can be sent via email or SMS.
For Zimmer Biomet, the result is a process where inspection is so close to production that it’s nearly single-piece flow.
“We take the part data from inspection on one part and feed it back in the machine to make the next one,” explains Livingston. “Say I’m going to make my first part of the day, and the line is empty. I’m going to manufacture my first part according to my setup sheet; from there I’m going to measure it and feed the data back into the machine to make part No. 2. So, if I don’t give them data for part No. 2, they can’t manufacture it. We just stop everything until we investigate what’s going on.”
Zimmer Biomet is effectively using Hexagon technology in a sort of virtual and on system. The technique has a positive result on reducing muda.
“The consequence of [QC being so close to manufacturing] is there’s no product between processes,” says Livingston.
“Comparing our facility five years ago to today, there’s a significant amount of work in progress (WIP) that’s not out on the floor anymore. We’re very lean now.”
FDA compliance
The U.S. Food and Drug Administration’s (FDA) Center for Devices and Radiological Health (CDRH) is responsible for regulating firms who manufacture, repackage, relabel, and/ or import medical devices sold in the United States. Part of Zimmer Biomet’s program for compliance to FDA regulations includes temperature, humidity, and vibration monitoring via the PULSE units.
“PULSE is kind of like a flight recorder that sits on each machine,” says Livingston. “First and foremost, it’s our compliance. The FDA ensures that you say what you do, and then that you do what you say you do,” explains Livingston. “if we say in a procedure we’re going to have the temperature for the CMM to be between 69 and 71 degrees, PULSE assures our compliance to that. We are required to have a statistical means for uncertainty, we have to have a protocol-driven approach to everything we do.”
The impact of utilizing PULSE technology on day-to-day manufacturing within a regulatory context is significant.
“The other day, we saw on the dashboard that around 4 a.m. there was a crash event on one of our CMMs, says Livingston. “By 7 a.m., with the data from PULSE and help from Hexagon’s field-service guys, we knew when the event happened and which products to quarantine because we have this time-date stamp. In the medical device field, containment is huge.”
Hexagon and Zimmer Biomet work together setting parameters that correspond to actionable commands.
“We can set maximum and minimum temp limits for an alert, so if the environment of any particular asset or CMM falls outside the specified parameters, we get an alert via email or text, and the action taken would be to just stop—stop taking measurements, stop running the measurement program, and
just keep our [manufacturing process] from creating a bad part,” explains Livingston. “Detection is valuable, but it’s even more valuable to never make a bad part. Now we have PULSE and we get alerts when our CMMs approach the programmed limits of temperature, humidity, or vibration. More importantly, we can just shut down and never make bad products, so my containment essentially goes away.”
ROI
When investing in manufacturing technology, the question of return on investment invariably comes into play.
“We had an event where a machine kept giving the operators errors,” explains Livingston. “Technicians would come in, and it would check out fine. We would return it to production, and it would fail again. This went on for about four weeks, and we ended up freighting the machine back to Rhode Island. The root cause was a hairline stress fracture in the bridge. If we had had a PULSE system at that time, we could have identified it that day. We’re averting so much trouble and downtime with PULSE.”
Unfortunately, the incident occurred during a holiday, resulting in inflated overtime costs to rectify the problem. Extrapolating how much time and money Zimmer Biomet has saved since installing may be difficult, but it’s safe to imagine a significant savings.
Future state of production
Zimmer Biomet co-manufactures with partners in multiple locations in the United States, Puerto Rico, Ireland, China, and Canada, employing hundreds of CMMs. If each machine is fitted with a PULSE system, the volume of data collected could be both a curse and a blessing. The curse is the inherent need for an ecosystem that can make sense of high-volume data. The blessing is that large quantities of data are rich with information—if you have the means to collate and cipher it.
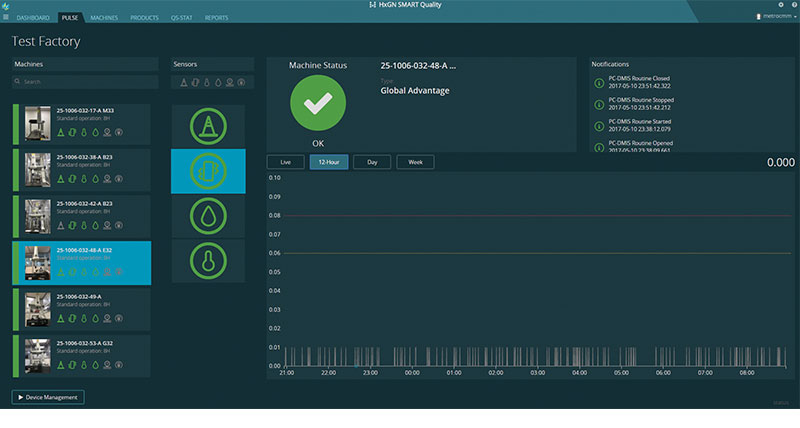
“For ease and to make data contextual and actionable, we brought in a tool called HxGN SMART Quality, developed by Hexagon, that networks the machines together,” says Livingston. “The live data feed [from PULSE boxes] is displayed every day in our lab on a 60 in. screen so our guys can look up and see what’s going on.”
In addition to live-feed capabilities, the HxGN SMART Quality solution also provides customizable reporting, which enables advanced data flow management and at-a-glance data visualization.
“You can run different machine reports depending on what you’re looking for,” says Livingston. “The contextual data are invaluable.”
With a forward-looking attitude, Zimmer Biomet embraces the Industrial Internet of Things (IIoT) concept wholeheartedly.
“The next thing becomes utilization and basically running your business tools,” says Livingston. “We have CMMs in the United States, Ireland, China, and Puerto Rico. With the HxGN SMART Quality system, we’re able to see the data coming from multiple PULSE boxes on one dashboard. With your machine utilization data available in that context, you can make much more informed decisions about how you run your business. For instance, can a facility in South America place a CMM in an open area, or does it have to be in a more environmentally controlled situation? Does a facility in China need more CMMs, or do they need to improve their processes?
“My future state is going to be more along the lines of getting a ‘last 10’ error report, or ‘what’s running in error right now,’ or ‘what’s getting close to error,’” says Livingston. “Instead of my guys running around putting out fires, they’ll be watching a dashboard and taking proactive measures based on data instead of crash alerts. It’s a whole new tool. We’re going into an actionable-data realm that just hasn’t been possible up to now.”
From humble beginnings in Justin Zimmer’s own basement to sponsoring the first-ever live internet broadcast of knee replacement surgery to computerized systems designed to help orthopedic surgeons, Zimmer Biomet has embraced continuous improvement and cutting-edge solutions. It will be interesting to follow this organization’s journey with Hexagon Manufacturing Intelligence.
“We’re still learning what the data can tell us and how we can use the data,” admits Livingston. “It has the potential to change how you decide on plant network optimization. It makes certain things that were insurmountable before very easily done.”
The other day, we saw on the dashboard that around 4 a.m. there was a crash event on one of our CMMs. By 7 a.m., with the data from PULSE and help from Hexagon’s field-service guys, we knew when the event happened and which products to quarantine because we have this time-date stamp. In the medical device field, containment is huge.